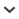Recent arachnid phylogenies support an Arachnopulmonata clade including scorpions, pseudoscorpions and the tetrapulmonate arachnids (i.e. spiders and their closest relatives). The position of the other arachnids is less certain, with molecular data suggesting that Arachnida may be paraphyletic with respect to horseshoe crabs. Here we explore the potential role fossil data can play in this debate. We outline the beneficial aspects of including fossils in phylogenies – fossils calibrate trees to time – as well as the challenges integrating these data. We tabulate the oldest occurrences of all major groups and superimpose these on recent phylogenetic hypotheses. Given that a key question is when (and how often) arachnids moved from water onto land, we review the early plant fossil record as a framework for when arthropod life on land may have been viable. In light of the aquatic ecology of horseshoe crabs, we then discuss the implications of placing this group within the arachnids, especially since some extinct lineages differ substantially from living species. In this context we re-assess what a horseshoe crab is from a palaeontological perspective, and speculate that some traditional Xiphosura fossils may actually lie on other parts of the euchelicerate tree. The oldest unequivocal horseshoe crabs are Ordovician in age (ca. 480 Ma), and probably predate complex terrestrial ecosystems. We conclude that recent phylogenetic results are best reconciled with fossils by inferring multiple terrestrialization events, possibly involving quite different approaches to breathing air. The lung-bearing (arachnopulmonate) branch of the tree is well resolved. Future work should focus on the apulmonate arachnids, and integrate the various early horseshoe-crab-like fossils into chelicerate phylogeny.
Neuere Arachniden-Phylogenien unterstützen eine Arachnopulmonata-Gruppe, welche die Skorpione, Pseudoskorpione und die Tetrapulmonata (d. h. Spinnen und ihre nächsten Verwandten) umfasst. Die Stellung der anderen Arachnidengruppen ist weniger gut gesichert, insbesondere weil molekulare Daten darauf hindeuten, dass die Arachnida in Bezug auf die Schwertschwänze (Xiphosura) paraphyletisch sein könnten. Hier untersuchen wir die mögliche Rolle, die fossile Daten in dieser Debatte spielen können. Wir skizzieren die positiven Aspekte der Einbeziehung von Fossilien in Phylogenien, denn Fossilien kalibrieren stammesgeschichtliche Bäume auf die Zeit, sowie die Herausforderungen bei der Integration dieser Daten. Wir stellen die ältesten Vorkommen aller wichtigen Gruppen tabellarisch dar und übertragen diese auf aktuelle phylogenetische Hypothesen. Da eine Schlüsselfrage darin besteht, wann (und wie oft) Spinnentiere vom Leben im Wasser zum Landleben übergegangen sind, ziehen wir das Wissen über frühe Pflanzenfossilien heran, um die Frage zu beleuchten, wann Arthropoden an Land leben konnten. Vor dem Hintergrund der aquatischen Ökologie der Schwertschwanze erörtern wir dann die Auswirkungen der Zuordnung dieser Gruppe zu den Spinnentieren, zumal sich einige ausgestorbene Linien erheblich von den lebenden Arten unterscheiden. In diesem Zusammenhang bewerten wir erneut, was ein Schwertschwanz aus paläontologischer Sicht ist, und spekulieren, dass einige traditionelle Xiphosura-Fossilien in Wirklichkeit auf anderen Teilen des Stammbaums der Eucheliceraten einzuordnen sind. Die ältesten eindeutigen Schwertschwänze stammen aus dem Ordovizium (ca. 480 Ma) und sind wahrscheinlich aus der Zeit bevor terrestrische Ökosysteme vorhanden waren. Wir kommen zu dem Schluss, dass die jüngsten phylogenetischen Hypothesen am besten mit den Fossilien in Einklang zu bringen sind, indem wir mehrmalige Evolution des Landgangs annehmen, die möglicherweise mit ganz unterschiedlichen Mechanismen der Luftatmung einhergingen. Der lungentragende (arachnopulmonate) Zweig des Stammbaums ist gut geklärt. Zukünftige Arbeiten sollten sich auf die Arachniden ohne Lungen konzentrieren und die verschiedenen frühen schwertschwanzartigen Fossilien in die phylogenetische Analyse der Cheliceraten integrieren.
Evolutionary relationships among arachnids and their relatives remain subject to controversy and debate (see Giribet 2018 for a recent overview). In recent publications a degree of consensus has emerged for a ‘pulmonate’ group (sensu Firstman 1973), which Sharma et al. (2014) formally named Arachnopulmonata. This clade comprises the lung-bearing arachnids: i.e. scorpions (Scorpiones), together with the tetrapulmonate arachnids – spiders (Araneae), whip spiders (Amblypygi), whip scorpions (Thelyphonida; some authors use Uropygi as the ordinal name) and schizomids (Schizomida). The extinct orders Trigonotarbida, Haptopoda and Uraraneida are also usually included in this grouping (e.g. Shultz 2007, Garwood & Dunlop 2014, Wang et al. 2018). Pseudoscorpions (Pseudoscorpiones) have been recovered as the sister group of scorpions in some analyses, forming a group named Panscorpiones by Ontano et al. (2021). This implies lungs are a plesiomorphic pseudoscorpion trait, and must have been replaced at some stage by tracheal systems: a transition that has also occurred several times independently within the spiders (Ramírez et al. 2021). Numerous, divergent lines of evidence now support Arachnopulmonata (although analyses of morphological data matrices do not), including: the morphology of the circulatory and visual systems (Klußmann-Fricke & Wirkner 2016, Lehmann & Melzer 2019); genomic changes involving whole genome duplications and the associated duplications of transcription factors (Nolan et al. 2020); and several independently conducted molecular phylogenies (e.g. Lozano-Fernandez et al. 2019b; Noah et al. 2020). For a review of the possible evolutionary transitions in the arachnopulmonates towards modern spiders see Dunlop (2022).
In contrast, the picture is less clear for the other arachnids, with the remaining orders best represented as a polytomy (e.g. Giribet 2018: fig. 4). This polytomy comprises palpigrades (Palpigradi), harvestmen (Opiliones), the extinct Phalangiotarbida, ricinuelids (Ricinulei), camel spiders (Solifugae) and the two main divisions of the mites, which are now usually referred to as Acariformes and Parasitiformes. All of these arachnids do not possess lungs, with palpigrades and several mites lacking respiratory organs altogether (although some authors suggested the ‘ventral sacs’ in prokoeneniid palpigrades may have respiratory origins; Kaestner 1956, Alberti et al. 1992). While it is tempting to recognise a corresponding ‘apulmonate’ group of arachnids (sensu Firstman 1973), when present the trachea across this assemblage of arachnids open on different body segments, and may thus not be serially homologous; see also comments in Kraus (1998). Current debates in this part of the tree include whether mites are monophyletic – compare, for example, Lozano-Fernandez et al. (2019b) and Ontano et al. (2022). If Acari is not a clade then what are the sister groups of acariforms and parasitiforms respectively? Related to this, the position of the palpigrades is of interest given that they show several plesiomorphic traits for arachnids (Savory 1974). Molecular data found some support for a sister group relationship with camel spiders (Ballesteros et al. 2019), while the detailed morphological study of Franz-Guess & Starck (2020) identified characters suggesting affinities with acariform mites; see also the molecular analysis of Noah et al. (2020). There is also some morphological evidence for placing ricinuleids close to the extinct trigonotarbids (Dunlop et al. 2009), which could potentially draw another trachea-bearing group of arachnids into Arachnopulmonata. In phylogenomic analyses the Ricinulei are recovered in various positions depending on matrix composition and analytical approach (e.g. Ballesteros et al. 2022).
One of the most interesting results in the recent literature has been the proposal that arachnids are not monophyletic (Ballesteros & Sharma 2019, Ban et al. 2022), specifically through the horseshoe crabs (Xiphosura) nesting within the traditional Arachnida. This hypothesis has not gone unchallenged (Howard et al. 2020), but the result has been robustly defended (Sharma et al. 2021; Ballesteros et al. 2022) – the latter studies entertaining the possibility of arachnids having undergone several independent colonisations of the land. Our aim herein is not to adjudicate between divergent molecular phylogenies, and nor can we answer whether arachnids (excluding the horseshoe crabs) are a clade. Instead we would like to explore how fossils (Figs 1-2) and a deep time perspective (Figs 3-4) helps us understand the early stages of arachnid/chelicerate evolution. We focus on four topics. First, where lie the challenges in resolving chelicerate and arachnid phylogeny? Second, what are the minimum divergence times of the major groups? Third, does an understanding of the broader framework of when life on land was possible help assess when, and how often, arachnid terrestrialisation events may have occurred? Finally, since current debates focus on the position of the Xiphosura it is worth asking what, in a paleontological context, do we mean by a horseshoe crab?
Methods
Data were reviewed from the literature as well as our own examinations of several key fossils (Figs 1-2) over the years; see e.g. Garwood & Dunlop (2014), Garwood et al. (2016).
Results and Discussion
Why consider fossils?
There are numerous reasons to include extinct taxa in phylogenetic analyses (Mongiardino Koch & Parry 2020). Recent research using simulations demonstrate that fossils improve the accuracy of phylogenetic inference, and increase the number of resolved nodes (Mongiardino Koch et al. 2021). That study also demonstrates that fossils can induce the collapse of nodes that are typically incorrectly resolved in analyses comprising just extant taxa – in particular, those around deep, short internodes representing ancient rapid radiations. Extinct taxa also provide unique insights into character acquisition on the stem group of a clade. They reveal, for example, that spigots (Fig. 2b) probably evolved prior to spinnerets on the spider stem group (Selden et al. 2008). They can preserve character combinations that are not present in living groups. For instance, Chimerarachnida, a putative sister group to all other spiders (Fig. 2g), possessed both spinnerets and a long, flagelliform tail (Wang et al. 2018, Huang et al. 2018). Furthermore, fossils provide temporal constraints to phylogenies. In doing so, they facilitate morphological and molecular clock analyses (Warnock & Wright 2021), and thus clade origin times and analyses of rates.
Whence challenges?
As such, the inclusion of fossil chelicerates can play a key role in understanding the phylogeny of a group, as well as the timing and nature of its evolution. However, it is not a silver bullet, and phylogenetic inference remains challenging, especially in light of the evolutionary history of chelicerates. For instance, ancient rapid radiations of the kind we might expect following terrestrialisation and adaptation into early terrestrial niches (be there single, or multiple instances in the arachnids) are difficult to reconstruct through phylogenetic inference (Whitfield & Kjer 2008). This is true of both morphological and molecular phylogenies: such an event results in short branches, deep in a phylogeny, upon which few characters might be expected to accumulate, and those that do are overprinted by character changes on the longer branches they subtend. This could be a prominent factor in the lack of resolution surrounding many key nodes (see again Giribet 2018) in the relationships between the arachnid/chelicerate orders. It also dictates, however, that when using phylogenetic comparative methods to understand arachnid evolution, these relationships will have limited impact on the results (Harmon 2019). Furthermore, the oldest putative member of the extant horseshoe crab genus Limulus is Jurassic (Fig. 1f), while molecular clock studies suggest that the xiphosurid crown-group (i.e. the living genera) split early in the Cretaceous period (Obst et al. 2012). As a result, those horseshoe crabs for which we have molecular data are themselves already situated on a long (>250 Ma) branch. A combination of short and long branches can be challenging for phylogenetic inference (Felsenstein 1978), even if rates of evolution are equal between lineages (Bergsten 2005): most notably in parsimony analyses of the kind typically used for morphology. This could contribute to the difficulty placing horseshoe crabs accurately in morphological phylogenies restricted to extant taxa. We note, however, that Xiphosura are relatively stable in phylogenomic analyses, as demonstrated by taxon deletion experiments (Ballesteros & Sharma 2019: fig. 5), and thus this branch is not amongst the problematic ones in such chelicerate phylogenies.
Fig. 1.
a. casts of impressions in the Upper Cambrian (Dresbachian) Hickory Sandstone of Texas, USA, interpreted as resting traces of a Chasmataspis-like animal (Dunlop et al. 2004); b. the stem-Xiphosurida Lunataspis aurora, Ordovician Churchill River Group, Canada (Rudkin et al. 2008, image from Bicknell & Pates 2020); c. a Silurian eurypterid, Eurypterus remipes, from Fiddlers Green Formation, USA (Tetlie et al. 2007); d. a digital reconstruction of the synxiphosurine Dibasterium durgae from the Silurian Herefordshire Lagerstätte, UK (Briggs et al. 2012); e. another synxiphosurine from the Herefordshire Lagerstätte – Offacolus kingi (image courtesy of Mark Sutton, fossil ∼5 mm); f. Limulus darwini, the oldest known member of this genus from the Late Jurassic of Owadów-Brzezinki, Poland (Błażejowski 2015); g. the SIlurian synziphosurine Bunodes lunula, Oesel Group, Saaremaa Island, Estonia (Bicknell & Pates 2020); h. Limuloides limuloides, a Silurian synziphosurine from Leintwardine Formation, UK (Bicknell & Pates 2020); i. an X-ray negative showing Weinbergina opitzi from the Devonian Hunsrück Slate, Bundenbach Germany. All scale bars 10 mm

When fossils are included, the incomplete nature of their record provides another challenge for resolving chelicerate phylogeny. This is most prominent in terrestrial (i.e. arachnid) orders, where taphonomic biases result in long ghost lineages: these can be estimated via comparison with molecular clocks, which suggest, for example, a >150 Ma ghost lineage for the scorpion crown group (Howard et al. 2019). As a result, plesiomorphies may have been lost in even the earliest fossils, limiting their explanatory power. Similarly, a lack of suitable terrestrial Lagerstätten from the relevant time periods leaves a fossil record which does not sample the stem-group of the majority of supraordinal arachnid clades, and some of the orders. For example, there are hints that palpigrades retain several plesiomorphic characters, and thus might have branched off quite early from the other arachnids, but none of the current Palaeozoic Lagerstätten preserve them as fossils. Taphonomic biases, such as the fact that smaller arachnids tend to be preserved more frequently once amber deposits become available, and that e.g. genitalic characters are less commonly preserved, dictate that fossils have non-random missing data. This is not inherently problematic, but necessitates larger matrices and wider taxonomic sampling (Wiens 2003, Vernygora et al. 2020).
Fig. 2.
a. the opilionid Eophalangium sheari from the Devonian Rhynie Chert of the UK (Dunlop & Garwood 2018). Scale bar 1 mm; b. cuticle fragments of Attercopus fimbriunguis from Devonian of New York, showing spigots and a potential silk strand (top), and portions of a flagellum (bottom; Selden et al. 2008). Scale bars 0.5 mm; c. a Carboniferous whip spider, Weygoldtina anglica, from Coal Measures deposits of the West Midlands, UK. Scale bar 5 mm; d. a trigonotarbid arachnid – Palaeocharinus sp. – from the Rhynie chert (Dunlop & Garwood 2018). Scale bar 1 mm; e. a Devonian scorpion, Waeringoscorpio hefteri, from the Lower Devonian of the Rhenish Massif of Germany (Poschmann et al. 2008). Scale bar 5 mm; f. a yet to formally be described mite (Arachnida: Acariformes) from the Rhynie Chert (Dunlop & Garwood 2018); g. Chimerarachne yingi – a Cretaceous fossil from Burmese amber, which possessed both a flagelliform telson and spinnerets (Wang et al. 2018). Scale bar 1 mm; h. the Carboniferous spider Protolycosa suazoi, Kinney Brick Quarry, New Mexico, USA (Selden 2021). Scale bar 5 mm

Finally, the lack of molecular data for fossils necessitates their inclusion in either morphological (Shultz 2007, Garwood & Dunlop 2014) or total-evidence phylogenies (Ballesteros et al. 2022), both of which lack nuanced models to describe morphological evolution (Keating et al 2020). Hence, the inclusion of chelicerate fossils in phylogenies is desirable, but requires that we remain cognisant of the uncertainty inherent in these analyses. In future, these might be reduced by: advances in fossil analysis, such as the widespread use of X-ray microtomography (Figs 1d, e, 2c) for three-dimensionally preserved fossils (Garwood et al. 2014); through to the development of more comprehensive total-evidence analyses with new models; and via future fossil discoveries.
Dating divergence
The first, and most obvious, role of fossils in phylogeny is to calibrate trees to time, in particular by providing minimum dates for the appearance of individual groups. The traditional model of chelicerate evolution, drawing on Weygoldt & Paulus (1979), would be a hypothesis of the form (Pycnogonida (Xiphosura ((Eurypterida + Arachnida)))). We note that this sequence remains most consistent with the current fossil record (Tab. 1). The oldest putative sea spider comes from the late Cambrian, the oldest putative horseshoe crab is early Ordovician, the oldest eurypterid is mid Ordovician and the oldest arachnids are mid Silurian. Of course, this does not invalidate alternative topologies, see e.g. comments in Sharma et al. (2021), but this temporal component could provide valuable data (Budd & Mann 2020). For example, the sister group of the horseshoe crabs is implicitly at least as old as their oldest fossils and, as we will argue below, this will impact the types of environment it could have lived in.
In this context we offer a list (Tab. 1) of the oldest records for all major groups in the Chelicerata, updated from previous summaries in Dunlop (2010) and Wolfe et al. (2016). The oldest putative pycnogonid comes from the ‘Orsten’ fauna of Sweden (Waloszek & Dunlop 2002). Pycnogonid affinities were not accepted by Bamber (2007), although other workers have been more sympathetic towards the hypothesis that it is an early developmental instar of a sea spider, or at least an arthropod very close to sea spider origins (Brenneis et al. 2017). The antiquity of this fossil is in keeping with molecular clock studies that recover Late Cambrian to Early Silurian age estimates for the pycnogonid crown group (Ballesteros et al. 2021), although the oldest unambiguous sea spider is the Silurian species Haliestes (Siveter et al. 2004). Chasmataspidida are an extinct group which were initially interpreted as unusual horseshoe crabs. Their putative Cambrian record (Dunlop et al. 2004) is based on resting impressions from the Hickory Sandstone of Texas (Fig. 1A), which is not well-constrained stratigraphically, but is thought to be late Cambrian. However, we should cation that Braddy & Gass (2023) recently suggested that these impressions may have been made by phyllocarid crustaceans and the oldest unequivocal chasmataspidid body fossils are Ordovician. As discussed below, we make a distinction herein between Xiphosura as a broad group containing all putative horseshoe crabs and the more restricted Xiphosurida clade; see e.g. Bicknell & Pates (2020) or Lamsdell (2020) for details. Xiphosura as a whole originated in the Ordovician (Van Roy et al. 2010), but on current data Xiphosurida – as defined by Lamsdell (2020) – first appear in the Late Devonian based on the Russian species Bellinuroopsis rossicus Chernyshev, 1933. We note, however, that the Xiphosurida lineage, if we consider this group to compose Xiphosura with fused opisthosomal tergites, either has its roots in the Ordovician (Rudkin et al. 2008), or else this thoracetron-like arrangement of fused tergites has evolved convergently in more than one lineage.
Tab. 1:
Summary of the oldest fossil records for the major clades of chelicerates, including the arachnid orders

The majority of the remaining crown-group arachnid orders either first appear in the Devonian, such as Opiliones (Fig 2A), Pseudoscorpiones, Acariformes (Fig. 2F) and Uraraneida (Fig. 2B), or in Carboniferous Coal Measures Lagerstätten such as Ricinulei and most of the tetrapulmonates (Figs 2C, 2H). Molecular clock estimates suggest the origins of many groups significantly predate their appearance in the fossil record. For instance, there is a gap of 30–95 Ma between molecular clock estimates for spider origins (Fernández et al. 2018, Magalhaes et al. 2020) and the first undisputed mesothele (Selden et al. 2014). We note that the young (Cretaceous) age for the earliest parasitiform mites, palpigrades and schizomids is probably a taphonomic artefact; Palaeozoic origins are implied by the vast majority of potential arachnid topologies. The oldest formally described parasitiform mites come from Burmese amber, but there are putative records from slightly older Lebanese amber mentioned by Rasnitsyn et al. (2016). As a working hypothesis (Fig. 3), we combined the combined molecular and morphological phylogenetic tree of Ballesteros et al. (2022: fig. 5C) with the dates in Tab. 1.
A framework for terrestrialisation
Living horseshoe crabs are a marine group, with one species able to enter estuarine environments. They also crawl onto shore to mate and lay their eggs. If this behaviour is plesiomorphic for horseshoe crabs it might hint at how some early chelicerates began the transition onto land. As noted above, there is evidence that horseshoe crabs may have originated within the arachnids, and there is also evidence that at least some early scorpions may have been aquatic; a hypothesis promoted by Kjellesvig-Waering (1986) in particular and critically reviewed by Howard et al. (2019). Some Devonian scorpions even reveal structures interpreted as external gills (Fig. 2E), with a suggestion that these species could be secondarily aquatic (Poschmann et al. 2008). An appreciation of when, and how often, arachnids shifted from aquatic to terrestrial ecosystems is thus intimately tied to our understanding of their early evolution, and informed by their phylogeny. Comparing the chelicerate fossil record (Tab. 1) with a framework for when life on land may have been possible (see also Buatois et al. 2022) could be helpful for assessing alternative hypotheses. In this context, an excursion into the history of potential primary producers could be informative.
The earliest terrestrial animals might have been supported by an extensive microbial cover. This predates animal life on land, and may have been forming soils as early as ca. 850 Ma (Wellman & Strother 2015, McMahon et al. 2021). It is thus possible that early land arthropods could have contributed to saprophagy-based ecosystems. We note that there is also precedent for modern arthropods living in intermediate environments, such as sandhoppers in seaweed washed up on the shoreline, mites and other tiny arthropods living in damp interstitial spaces between soil particles (Dunlop et al. 2013), and palpigrades in the interstices of sand in tidal waters (Condé 1965). Drawing on ideas developed by Ghilarov (1958), van Straalen (2021) reviewed the evidence that soils represented a key evolutionary transition zone between entirely aquatic and entirely terrestrial environments, and was thus a likely route onto land for several groups of worms and arthropods (see also below). Yet since plants form the basis of most modern terrestrial ecosystems it is tempting to assume that a degree of plant cover was a prerequisite for arthropods fully colonising the land.
A recent overview of early plant evolution can be found in Morris et al. (2018). The terrestrial vegetation record shares several issues with that of the arachnids, namely a lack of consensus about the first branching events, and a sparsity of fossils during the earliest phases of plant evolution which could document the sequential acquisition of characters. Palaeobotanists are thus drawn towards reliance on molecular data too. Summarising Morris et al.'s (2018) results, land plants (i.e. embryophytes) are estimated to have arisen sometime between the mid Cambrian and the early Ordovician (ca. 515.2 to 473.5 Ma), with the vascular plants (tracheophytes) evolving a little later from the late Ordovician to the Silurian (ca. 472.2 to 422 Ma). These molecular estimates are broadly compatible with the fossil record which includes so-called cryptospores of uncertain affinity from the Cambrian, tetrahedral cryptospores – which are more convincing evidence of embryophytes – from the mid Ordovician, and putative vascular plants from the Silurian onwards. Strother & Foster (2021) also described ca. 480 Ma spores which appear to be intermediate between the earlier spores of uncertain phylogenetic/ecological affinity and unequivocal land plant spores found later.
These data suggest that terrestrial plants at the organisational level of hornworts, liverworts or mosses (i.e. bryophytes) could have been present as early as the Cambrian, but that the vascular plants such as ferns or clubmosses did not appear before the end of the Ordovician. A key question is, how much plant cover/diversity is necessary to support a viable terrestrial arthropod community? Capel et al. (2022) hypothesised a Silurian–Devonian terrestrial revolution during which the vascular plants diversified and came to dominate the landscapes. Unequivocal terrestrial myriapods and arachnids are also Silurian in age (Jeram et al. 1990, Selden 2019) and the oldest unequivocal hexapods are Early Devonian (reviewed by Dunlop & Garwood 2018).
At the same time, we note that Capel et al.'s (2022) proposal is based on the known plant body fossil record and that the molecular data imply radiations may have occurred earlier. The same is potentially true of arachnids, namely that earlier occurrences have not (yet) been documented. This could, for example, be due to a sparsity of appropriate fossil localities, or because early members of the group had a low preservation potential due to their size or ecology. In this context, when considering terrestrialisation within arachnids, we note that the first appearance dates for terrestrial clades are clearly clumped based on Lagerstätten effects. For example, harvestmen and mites appear together in the early Devonian Rhynie chert (Figs 2A, F) and pseudoscorpions and uraraneids (Fig. 2B) in the slightly younger Gilboa ecosystem. We might expect this Lagerstätten effect to be weaker with trace fossils: there are numerous deposits that record tracks from the early terrestrial arthropod fauna (see Buatois et al. 2022 for a recent synthesis), and good coverage of land ecosystems prior to this point. Despite this, non-aquatic arthropod trace fossils do not significantly predate the appearance of body fossils (Minter et al. 2017). This could provide support for terrestrialization timings that are more closely aligned with the fossil record than molecular clocks. We should caution that some Ordovician trackways may actually be examples of ‘death traces’ produced by animals which came onto shore accidentally (Shillito & Davies 2019), rather than evidence of habitually terrestrial animals. Lack of trace fossil evidence might reflect very small body sizes in early terrestrial arthropod lineages with (millimetre scale?) animals less likely to have been able to leave a preservable trackway in the sediment.
To summarise, current datasets allow the possibility of terrestrial chelicerates as early as the Cambrian, but questions remain regarding the extent to which these ecosystems could have supported a diverse arthropod community, and the nature of their primary producers. The Silurian fossil record of arachnids (Tab. 1), in their traditional sense, appears to correspond with a proposed radiation of the vascular plants (Fig. 1), perhaps offering an increasing diversity of microhabitats and food sources for primary consumers. Early arachnids must have eaten something. It is noteworthy that the extant palpigrade Eukoenenia spelaea feeds on cyanobacteria (Smrž et al. 2013); showing that a diet which would have been viable in even the earliest terrestrial ecosystems, before the origin of plants, exists in some living taxa. Assuming, however, that the majority of early arachnids were predators (as with the extant species) and considering the groups on which modern arachnids can (or do) feed, Myriapoda present a similar situation (Brookfield et al. 2021), i.e. origins inferred from molecular data in the Cambrian, but no unequivocal terrestrial record prior to the Silurian. For hexapods, their putative sister group are crustaceans in the Xenocarida group, which thus also imply Cambrian origins (Wang et al. 2016), but as noted above there are no fossils until the Early Devonian. In both cases the authors suggested that fossils filling these gaps may be discovered, albeit in marine, possibly meiofaunal, sediments. Indeed, for myriapods the recent recognition that the enigmatic euthycarcinoids, which first appear in the Cambrian, are probably stem myriapods (Edgecombe et al. 2020) which may have later developed terrestrial adaptations (Gueriau et al. 2020) helps bridge the gap between the initial radiation of arthropod body plans during the Cambrian Explosion and the subsequent invasion of the land. An interesting question, of course, is what did the arachnid common ancestor look like. To answer this, we need to know which animals belong in this clade.
Fig. 3.
Two recent chelicerate phylogenies with the fossil record superimposed. Green box indicates the Silurian–Devonian terrestrial revolution sensu Capel et al. (2022), a time period where several arachnid orders first appear in the fossil record. Divergence estimates for embryophytes and tracheophytes, displayed as green range bars, are based on the work of Morris et al. (2018). Top. The Maximum likelihood tree of Ballesteros et al. (2022: 5C), combining molecular data, morphology and fossils, superimposed on the fossil record based on Tab. 1. Note how the early appearance of horseshoe crabs and chasmataspidids implicitly drags several lineages of ‘apulmonate’ arachnids down into the Cambrian and creates large ghost ranges during which the groups should have been present. Bottom. An alternative scenario, using a topology largely based on Lamsdell (2013, 2016, 2020), with a focus on the horseshoe crabs. Here arachnid monophyly was assumed, but the key message is that the traditional concept of the horseshoe crabs has been dismantled. Some horseshoe-crab like fossils with several pairs of biramous prosomal limbs were placed at the base of the euchelicerates, with others closer to either Xiphosura or to the lineage leading to arachnids. Interestingly, there is a notable concentration of so-called synziphosurine taxa at about the time of the inferred Silurian–Devonian terrestrial revolution (green box)

Implications of horseshoe crabs being arachnids
In 1881 E. Ray Lankester published his classic study “Limulus an Arachnid” in which he convincingly demonstrated that horseshoe crabs are closer to arachnids than to crustaceans. In fairness, other workers had implied this prior to Lankester (1881), but the detailed comparisons presented in his paper is rightly regarded as a paradigm shift in our understanding of arthropod evolution. Traditionally, a broad split into a marine Merostomata (i.e. horseshoe crabs and eurypterids) and a terrestrial Arachnida was recognised, although as noted by Kraus (1976) this is an ecological division rather than a phylogenetic one. While arachnids have been broadly accepted as a natural group, there have been occasional attempts to break their monophyly. Given the scorpion-like habitus of several eurypterids – hence their common name of ‘sea scorpions’ – there have been a number of claims (e.g. Pocock 1901, Versluys & Demoll 1920, Størmer 1963, Kjellesvig-Waering 1986, Dunlop 1998) that they are closely related to, or even gave rise to, the scorpions. Cladistic analysis of morphological characters (Shultz 2007; Garwood & Dunlop 2014, Ballesteros et al. 2022) has not supported this hypothesis. Van der Hammen (1985) proposed a Myliosomata group including horseshoe crabs, harvestmen and scorpions, which he united on a similar mode of feeding involving projections from the coxae. This specific grouping has not been supported by any subsequent studies and illustrates the weakness of proposing major clades based on a single character system.
The strongest evidence for the non-monophyly of arachnids derives from modern molecular data, recently combined with morphological data in a total evidence approach (Ballesteros et al. 2022), which recovered horseshoe crabs within Arachnida. A summary of this, alongside critiques of morphological support for the traditional concept of Arachnida, have been set out in considerable detail by Sharma et al. (2021). Xiphosura have been resolved either as the sister group of Ricinulei (Ballesteros & Sharma 2019, Ballesteros et al. 2022) or as the sister group of Arachnopulmonata (Noah et al. 2020). From a palaeontological perspective what is interesting here is what these results imply (Fig. 3). As noted above, the oldest horseshoe crabs come from the Ordovician (Tab 1). If Arachnida (including Xiphosura) is monophyletic then their common ancestor must have been at least Early Ordovician (ca. 480 Ma) in age, and thus predated the appearance of the vascular plants which were probably absent prior to ca. 470 Ma. We thus find scenarios in which Arachnida was supposed to have had a single terrestrial common ancestor, and in which horseshoe crabs re-entered the water secondarily (e.g. Noah et al. 2020) challenging to reconcile with the fossil record. In these hypotheses the basal splits among the arachnids sensu Ballesteros & Sharma (2019) should have taken place (Fig. 3) at a time when it is questionable whether there was any animal life permanently inhabiting the land. As noted above, a fully-developed terrestrial ecosystem may not have been present before the Silurian at ca. 420 Ma (cf. Buatois et al. 2022, Capel et al. 2022).
There are also significant morphological implications of including horseshoe crabs within the arachnids. We note the argument – both in the context of arachnid relationships, and phylogenetics as a whole – that morphology no longer has a role to play in phylogenetic reconstruction in the era of phylogenomics (Sharma et al. 2021). If we are to include fossils in our analyses to provide insights into character acquisition, timings, or evolutionary rates, however, the consideration of morphology remains necessary. Furthermore, we note that arachnid clades are still often named and recognised based on key morphological innovations (Cephalosomata/Arachnopulmonata/Tetrapulmonata/Pedipalpi), and suspect that the least controversial parts of the arachnid tree (Giribet 2018) such as Tetrapulmonata have achieved broad acceptance precisely because molecular data are supported by a suite of convincing evolutionary novelties for which a logical sequence of character acquisition can be reconstructed (reviewed by Dunlop 2022). In this case, there is a ground pattern of four book lungs, similar ‘clasp-knife’ mouthparts and a narrowing between the prosoma and opisthosoma. To the best of our knowledge, there are no obvious morphological synapomorphies for, e.g., (Xiphosura + Ricinulei). That this has little morphological support does not invalidate the result as any putative relationship implies some homoplasy. Indeed, there are numerous sister group relationships based on molecular results that are broadly supported, but have little in the way of morphological support: Remipedia + Hexapoda is an excellent example (Lozano-Fernandez et al. 2019a). Grouping taxa with radically different anatomies and ecologies such as this is thus an excellent avenue for further investigation, in search of potential evolutionary explanation.
Another implication of the inclusion of horseshoe crabs within the arachnids is found in the well-preserved fossils of putative horseshoe crabs like the Silurian Dibasterium durgae Briggs et al., 2012 (Fig. 1d) which express character combinations very different from living Xiphosura. Dibasterium durgae is remarkable for retaining a series of biramous prosomal limbs behind the chelicerae (Briggs et al. 2012), as opposed to the small flabellum emerging from the coxa of the last pair of legs in living horseshoe crabs. Biramous limbs are widely accepted by palaeontologists as having characterised the last common ancestor of all arthropods; for recent summaries and possible evolutionary scenarios see Fu et al. (2021) and Liu et al. (2022). If Dibasterium durgae is a horseshoe crab, as proposed in Briggs et al.'s original description, and if horseshoe crabs are arachnids, it implies that a fully developed series of prosomal biramous limbs is a plesiomorphic trait for the Arachnida. As such, this necessitates multiple convergent losses of biramy both within Xiphosura and within several clades of arachnids. Alternatively, and perhaps less plausibly, the common ancestor of arachnids and horseshoe crabs could have had largely uniramous prosomal limbs with biramy (re-) evolving in a few extinct genera.
Fig. 4.
A possible solution towards reconciling recent molecular results with fossil data; see text for details. Arachnida (including horseshoe crabs) could have undergone at least two independent terrestrialization events. The less well resolved apulmonates may have been a more ancient radiation of smaller animals which came onto land via early soil ecosystems where they mostly evolved trachea. The better resolved arachnopulmonates may have come onto land later (and larger) via a more direct route and transformed book gills into book lungs. Several putative horseshoe crabs (the synziphosurines) may actually represent stem-lineages of other major clades, while the position of the eurypterids and chasmataspidids merits further study. Green box again shows the Silurian–Devonian terrestrial revolution

But is Dibasterium durgae really a horseshoe crab? This is not a trivial question as it impacts profoundly on our understanding of the Xiphosura ground pattern: i.e. biramous or uniramous prosomal limbs? Among recent studies Bicknall & Pates (2020) listed D. durgae as a xiphosuran, albeit one of uncertain suprageneric affinities, but Lamsdell (2016, 2020) chose not to include this unusual-looking species in his comprehensive xiphosuran phylogenies.
So what is a horseshoe crab?
This leads us to a key question: what do we mean, in palaeontological terms, when we talk about horseshoe crabs? In addition to the three living genera, these animals also have a long and rich fossil record (Bicknell & Pates 2020, Lamsdell 2020). Traditionally, horseshoe crabs were divided into two groups: the extinct synziphosurines and the Xiphosurida (true horseshoe crabs) which include several fossils as well as the living horseshoe crabs. As noted above, Xiphosurida were defined by all tergites of the opisthosoma being fused into a single plate: the thoracetron. Over the years synziphosurines (Fig. 1g-i) became something of a waste-basket for Palaeozoic fossils resembling living horseshoe crabs. In a notable paper Lamsdell (2013) suggested the Xiphosura were not monophyletic. His basic proposal was that synziphosurines in fact represent several grades of organisation, and include lineages which are close to horseshoe crabs (the Xiphosura sensu stricto) and potentially also to the eurypterids and/or arachnids.
In detail, Lamsdell (2013, 2016) recognised a series of nested clades (Fig. 3) within the broader Euchelicerata, i.e. all chelicerates excluding sea spiders. The enigmatic Silurian fossil Offacolus kingi Orr et al., 2000 (Fig. 1e) which also has biramous prosomal limbs (Sutton et al. 2002), was placed at the base of the euchelicerates. In Lamsdell (2016) Dibasterium durgae occupied a similar position (a position broadly equivalent to that recovered in some arachnid-wide phylogenies, e.g. Wang et al. 2018, Huang et al. 2018). These genera are followed by a clade named Prosomapoda in which prosomal limbs II–V are no longer biramous. This grade of organisation includes several synziphosurine genera. One branch led to the Xiphosura, defined here explicitly on characters such as a prosoma bearing a cardiac lobe and ophthalmic ridges forming double arch, 7th appendages reduced to chilaria and a partially reduced tergite of the 7th somite. Another branch led to a clade named Planaterga in which the tergites lack axial nodes (i.e. they lack raised, median structures on the posterior tergal margin). This clade includes several further synziphosurines, as well as the Dekatriata including chasmataspidids, eurypterids and (in this scenario) a monophyletic Arachnida. Dekatriata was defined on an opisthosoma of 13 segments in the ground pattern. A phylogeny along these lines requires fossils, and thus the inclusion of morphology. It suggests that fossils traditionally assigned to horseshoe crabs are probably not a homogeneous group and may resolve on different parts of the overall euchelicerate tree.
Thus a key challenge for future phylogenies is reconciling molecular results which place Xiphosura within Arachnida with the available diversity of xiphosuran-like fossils. Do some of the species previously interpreted as synziphosurines fall on the stem lineage of Euchelicerata, or even Chelicerata? Fossils with biramous prosomal limbs like Dibasterium durgae and Offacolus kingi (Fig. 1d-e) would certainly be candidates, although these interpretations are hindered by a lack of consensus about which Cambrian fossils are either chelicerates or their immediate outgroups. By way of example, Chen et al. (2004), Legg (2014) and Aria & Caron (2019) offer alternative candidates for the oldest chelicerate, all with quite different morphologies. Other synziphosurines may indeed belong on the stem lineage of Xiphosura, but others may be closer to, for example, eurypterids or perhaps even one or more arachnid lineages. Other enigmatic fossils have been proposed as possible intermediates between horseshoe crabs and eurypterids (e.g. Lamsdell et al. 2015b, Selden et al. 2015) and need to be integrated into the overall picture.
As noted above, we lack an equivalent to the euthycarcinoid/myriapod situation; namely a series of extinct animals which document the appearance and acquisition of characters in one or more arachnid clades. Perhaps such fossils do exist and have been hiding in plain sight (like the euthycarcinoids) among the synziphosurines? It is interesting to note that many synziphosurines are Silurian in age (Fig. 3), and thus contemporary with both the oldest arachnids and with the Silurian–Devonian terrestrial revolution of Capel et al. (2022). Even if the synziphosurines themselves were primarily aquatic, we might speculate whether their radiation coincided with one or more lineages attempting the transition onto land. Unfortunately, most synziphosurines are preserved as dorso-ventral compressions and often lack key characters of the limb series and respiratory organs with which to test these hypotheses. Virtual palaeontology, using techniques such as computed tomography (Sutton et al. 2014), could play a future role in revealing hidden anatomical details. Even if this scenario were accurate, we still lack fossils documenting the transition of synziphosurine-like taxa onto land: fossils which – if discovered – are unique in their ability to resolve the deepest nodes in arachnid phylogeny.
While synziphosurines are predominantly known from the Silurian and early Devonian, it is perhaps worth noting that there are no Silurian xiphosurans. There are only two convincing Ordovician fossils referable to Xiphosura (see below) while other putative records of this age are probably misidentifications (Lamsdell 2020). Despite several seemingly suitable Silurian nearshore localities across several palaeocontinents, hosting animals like synziphosurines and eurypterids, Xiphosura sensu stricto only appear again in the fossil record towards the end of the Devonian with the family Kasibelinuridae and the first putative member of the more derived Xiphosurida. In the Carboniferous, members of this order diversified into the extinct Belinurina and the still living Limulina clades (e.g. Lamsdell 2016: fig 2). It is not immediately clear why Xiphosura effectively disappear from the fossil record during the Silurian and most of the Devonian, but it does imply that the main radiation of the horseshoe crabs post-dates the radiation of the arachnids.
Multiple terrestrialisation events?
If we exclude most of the synziphosurines from being horseshoe crabs sensu stricto, Xiphosura still potentially dates back to the Ordovician (ca. 480 Ma). This is because, in addition to the earliest synziphosurine from the Late Tremadocian Lower Fezouata Formation (Martin et al. 2016), there is a putative xiphosuran from the Upper Fezouata Formation (Van Roy et al. 2010). Both taxa await formal description. Furthermore, Lunataspis aurora Rudkin et al., 2008 (Fig. 1b) was reported from Upper Ordovician (ca. 445 Ma) shallow marine sediments in Manitoba, Canada. It has been resolved as sister group to all other xiphosurans (e.g. Lamsdell 2020) and possesses an opisthosoma with a fused thoracetron: the defining character of Xiphosura. We reiterate that Xiphosurida, i.e. the more restricted clade including the modern taxa amenable to molecular studies, only goes back as far as the Late Devonian (Bicknell & Pates 2020, Lamsdell 2020) and is thus younger than many of the arachnid orders. Still, if horseshoe crabs are ingroup arachnids then the Moroccan and Canadian fossils from the Ordovician imply that arachnids radiated before terrestrial ecosystems had become properly established.
Among mandibulate arthropods it is clear that several lineages have (or have attempted to) come onto land independently: i.e. myriapods and multiple pancrustacean clades of which the insects have obviously been the most successful (Dunlop et al. 2013). A plausible scenario, which would still be compatible with a non-monophyletic Arachnida, would be that arachnids (including horseshoe crabs) also underwent multiple terrestrializations; essentially the hypothesis proposed by Ballesteros et al. (2022). Indeed, multiple terrestrialisation events within arachnids have long been a subject of discussion (e.g. Pocock 1901, Manton 1977, 1978). As noted above, a broad split into pulmonate and apulmonate arachnids was recognised by Firstman (1973). It is tempting to view these as reflecting two separate terrestrialization waves, characterised by two very different solutions to the challenges of breathing air on land: either converting existing gills into lungs within a branchiate/pulmonate clade or developing novel tracheal systems, perhaps convergently given the openings in different groups on different parts of the body. Book lungs may actually have been the less effective solution, as evidenced by their (partial) replacement by trachea in more active spiders (Ramírez et al. 2021), pseudoscorpions, and also potentially in ricinuleids if they are a member of this clade (as a close relationship to Xiphosura would imply). Palpigrades emerge as a particularly interesting group for having several plesiomorphic character states, having been placed close to the Tetrapulmonata in some phylogenies (e.g. Shultz 1990), but having neither lungs nor trachea (notwithstanding a potential respiratory function for the ventral sacs, see above). This begs the question whether they had pulmonate or tracheate ancestors? This would benefit from further study.
What is perhaps striking is that the pulmonate branch is much better resolved than the apulmonate arachnids (Fig. 3). It is interesting to consider why. This could reflect more symmetrical rates of evolution across arachnopulmonates (P. Sharma, pers. comm). Alternatively, is this a group which came onto land more recently (the oldest scorpion is ca. 435 Ma, the oldest trigonotarbid 417 Ma), perhaps even in staggered waves? If arachnopulmonates only began to diversify into their currently recognised orders from the time of the putative Silurian–Devonian terrestrial revolution sensu Capel et al. (2022) onwards, then their sequential appearance in the fossil record (Fig. 3) actually suggests a reasonable match between molecular and fossil data. Only a single ancestral lineage, a kind of ‘protopulmonate’, would be needed with a ghost range extending back to the Cambrian/Ordovician.
By contrast, relationships outside the pantetrapulmonates are not well resolved (Giribet 2018). In Fig. 3 at least five arachnid lineages should have implicitly extended back into the Cambrian/Ordovician, necessitating significant gaps until they appear in the fossil record from the Devonian onwards. In other words in this hypothesis there should have been very early representatives of the lineages leading to palpigrades, parasitiform mites, acariform mites/solifuges, harvestmen/ phalangiotarbids and ricinuleids. If apulmonates were a clade (as per Firstman 1973), as opposed to a grade, this would potentially reduce the number of ghost ranges. We caution, however, that neither morphology nor molecules currently recover a monophyletic Apulmonata. Despite this, exploring the origins of trachea could be an important step towards understanding early apulmonate evolution.
A possible scenario (Fig. 4) which would be consistent with the current data would be that apulmonate arachnids came onto land earlier, and perhaps more rapidly, than the arachnopulmonates. A very deep divergence might explain the difficulties in resolving apulmonate relationships towards one another, and in this context Ontano et al. (2022) discussed the fact that pseudoscorpions (an arachnopulmonate), palpigrades, acariform and parasitiform mites all have long branches in phylogenetic analyses. These destabilise trees through long branch attraction, in which rapidly evolving lineages become artificially grouped together, especially when subtended by short internodes due to a radiation of the kind we expect during terrestrialisation. It is also worth bearing in mind that apulmonate arachnids tend, on average, to be smaller than their arachnopulmonate relatives (Dunlop 2019) and it has been suggested that mites in particular have been tiny throughout their geological history (Sidorchuk 2018). As noted above, soils predated plants (Wellman & Strother 2015). If the apulmonates' ancestors came onto land via the soil route sensu van Straalen (2021) this might explain the long ghost ranges which reflect (a) their more ancient origins, perhaps even predating well-developed plant communities, and (b) the poor preservation potential of tiny arachnids living cryptically in semi-terrestrial soil ecosystems. We might further suggest that several soil-dwelling lineages developed trachea independently as they became increasingly less reliant on the wet spaces between soil particles.
Another speculative scenario would be that arachnoplumonates come onto land later (and larger) during one or more independent terrestrialization events, perhaps even associated with a behavioural predilection to mate and lay their eggs on land. Some of the Silurian synziphosurines, which are typically a few centimetres long (Fig. 1g-i), might thus be close to arachnopulmonate origins and with a larger body it may have been easier to convert external gills into internal lungs. In this context, another question which merits investigation is whether eurypterids (Fig. 1c) and chasmataspidids are closer to Xiphosura – effectively forming the traditional Merostomata – or to one of the arachnid branches. Given the presence of lamellate gills in eurypterids, perhaps even adapted for breathing air in some taxa (Lamsdell et al. 2020), close relationships to the arachnopulmonates might be intuitively more likely. Kamenz et al. (2011) offered evidence that sperm transfer in eurypterids was mediated by spermatophores, which is more arachnid-like than the simple release of sperm onto the eggs in close proximity to the female as seen in living horseshoe crabs and a possible preadaptation for mating on land. Like breathing air, other aspects of arachnid biology (reproduction, osmoregulation, locomotion) underwent fundamental changes during the transition onto land and could represent interesting case studies for convergent evolution.
All of this is, of course, contingent on the robustness of Xiphosura being ingroup arachnids. If further data continue to support this result then hypotheses of multiple arachnid terrestrializations will need to be developed further. Perhaps it will also be possible to begin to integrate some of the euchelicerate/synziphosurine fossils into these models, ideally filling the morphological and temporal gaps between the major evolutionary lineages within Chelicerata. Note added in proof: Simon Braddy (Braddy 2023) recently redescribed the putative Cambrian chasmataspid traces, and interpreted them as having been produced by phyllocarid crustaceans.
Acknowledgements
We thank Peter Michalik and Gabriele Uhl (Greifswald) for inviting this contribution as part of the symposium “Phylogeny and Evolution of Chelicerata”,Prashant Sharma (Wisconsin) for constructive discussions of current controversies relating to arachnid monophyly, Roger Benson (Oxford) for discussion of phylogenetic comparative methods, and Anthony Shillito (Oxford) for discussion of the terrestrial trace fossil record.We are grateful to Gonzalo Giribet (Harvard) and Prashant Sharma (Wisconsin) for insightful reviews which improved the manuscript.RJG was supported by the Natural Environment Research Council (grant no. NE/T000813/1).