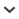We describe a new ant species from the north of the Iberian Peninsula, Myrmica babiensis sp. nov., a parasitic species of Myrmica aloba Forel, 1909. This new species is characterized by the exceptionally big body size, the lack of the carina or lobe in the base of the scape, the presence of conspicuous hairs on the eyes, and a very wide postpetiole. In this study, we also provide an illustrated key to all European parasitic Myrmica. Including M. babiensis sp. nov., the number of parasitic Myrmica species from the Iberian Peninsula rises to five. Additionally, we analyse the myrmecofauna of Babia and Luna, the mountainous regions where M. babiensis sp. nov. has been found, mostly composed of eurosiberian widespread species.
Introduction
Socially parasitic ants depend upon the labour of other ant species (the host) to obtain food, shelter, and care for their offspring (Buschinger 2009). Social parasites are phylogenetically closely related to the host and usually present isolated populations restricted to the area where their host is present (Hölldobler and Wilson 1990, Espadaler et al. 2010); and even there, the parasite density is very low (Baroni Urbani 1967). While social parasitism has been observed in various genera, the genus Myrmica Latreille, 1804 is well-represented, with twelve species showing parasitic habits (Radchenko and Elmes 2003, Barthi et al. 2016), being either temporary parasites or inquilines according to the classification by Hölldobler and Wilson (1990). Nevertheless, some parasitic Myrmica species have aroused particular interest due to their very low prevalence and the rarity of their records (e.g., Glaser et al. 2011).
To date, four parasitic Myrmica species have been recorded from the Iberian Peninsula:
1)Myrmica bibikoffi Kutter, 1963: distributed in Germany, Switzerland (Radchenko and Elmes 2010), France (Galkowski 2009), and Spain, where it is known from the provinces of Lleida and Pontevedra (García et al. 2008). Although some studies have reported the presence of workers of this species within the host nest, most records only involve solitary gynes. Therefore, it has been suggested that this species might be a temporary parasite and occasionally become an inquiline (Radchenko & Elmes 2010, Seifert 2018). The parasite is mainly associated with Myrmica sabuleti Meinert, 1861 in Central Europe (Radchenko and Elmes 2010), but in Spain this species has been confirmed from a colony of Myrmica spinosior Santschi, 1931 (García et al. 2008);
2)Myrmica karavajevi (Arnoldi, 1930): a widespread European workerless inquiline species (Radchenko and Elmes 2010). In Spain known from Lugo (García 2018) and Álava (Espadaler et al. 2004). This parasite uses as host several species of the scabrinodis group sensu Radchenko and Elmes (2010) (Seifert 2018). In the Iberian Peninsula, it has been recorded within the nests of Myrmica scabrinodis Nylander, 1846 (Espadaler et al. 2004) and Myrmica aloba Forel, 1909 (García 2018);
3)Myrmica lemasnei Bernard, 1967: the species is considered as a Pyrenean endemic. It was described from southern France and later recorded in Spain from Girona (García 2015) and Huesca (Espadaler 1981). It is a workerless inquiline parasite (Radchenko and Elmes 2010) that uses M. spinosior as a host species (García et al. 2008, García 2015). Although M. sabuleti was originally described as the host species, this information needs to be confirmed since it was published before the elevation of M. spinosior to the species level (Seifert 2005);
4)Myrmica vandeli Bondroit, 1920: a widespread European species distributed in humid habitats (Radchenko and Elmes 2010). It is known in Spain from two localities in the Pyrenees of Lleida (Espadaler 1986, Espadaler et al. 2010). Seifert (2018) presented an argumentation indicating that it is a facultative social parasite of Myrmica scabrinodis Nylander, 1846.
A recent record of Myrmica hirsuta Elmes, 1978 in southwestern France (Lebas and Galkwoski 2016) indicates the possibility for this species to be found in northern Spain in the future.
In this study, we contribute to the knowledge of social parasitism, by describing a new social parasite, Myrmica babiensis sp. nov., found within the nest of Iberian ant species M. aloba. Additionally, we also report new records of M. karavajevi in the Iberian Peninsula, include an updated map of the distribution of the five Iberian parasitic Myrmica species, and provide a taxonomic key that includes all the European parasitic Myrmica gynes.
Materials and methods
The first specimens were collected during a research project focused on studying the impact of traditional agricultural practices on the biodiversity conservation. This project was carried out in 2010 in 15 pastures associated to mountain passes located in the “Babia and Luna Natural Park”, León province (NW Spain), on the south side of the Cantabrian Mountains. During the study, we used plastic pitfall traps (88 mm depth, 65 mm diameter) partially filled with 25% propylene glycol and protected by 11 x 11 cm roofs of hardboard. In each mountain pass, we placed four groups of three pitfall traps (12 pitfall per pass) separated by approximately 50 meters. In total, we used 180 pitfall traps in the study. Three different treatments were considered, five replicates for each one: i) abandoned (not grazed in at least one year), ii) grazed by sheep, and iii) grazed by cows. The pitfall traps were replaced approximately every 20 days between the second week of July and the first week of October 2010. The collected material was segregated at the laboratory, and ants were separated from the rest of the collected material and stored independently in vials filled with 70% alcohol for further identification under a stereomicroscope. After the detection of the new parasite species in the collected material, we carried out several direct searches in the mountain passes in early October 2011, summers of 2012, 2016, 2021, and spring of 2022 to find specimens of parasites and confirm their host species.
The following biometric measurements and indexes were made using an ocular micrometer at a maximum magnification of 90×, and following Radchenko and Elmes (2003, 2010).
HL – Maximum length of the head in frontal view, from the anterior of clypeus to the medium point of occipital margin;
HW – width of the head in frontal view behind the eyes;
FW – minimal distance between frontal carinae;
FLW – maximum distance between the outer borders of the frontal lobes;
SL – Maximum length of scape, excluding the condylar bulb;
AL – mesosoma length, from most anterodorsal point to the posterior margin of propodeal lobes;
PH – height of petiole in profile view, perpendicularly to the line between anteroventral and posteroventral points of petiole;
ESL – in lateral view, length of propodeal spine, from the top to the deepest point of the propodeal constriction at the base;
ESD – distance between the tips of the propodeal spines, in dorsal view;
PL – length of petiole in dorsal view, from the posterodorsal margin of the petiole to the articulation with propodeum;
PPW – postpetiole width in dorsal view.
The distribution map of Iberian parasitic Myrmica was generated using the QGIS system software (QGIS 2022) using bibliographic and personally collected data (see examined material). The key to European parasitic Myrmica gynes was made by combining the information included in Radchenko and Elmes (2003, 2010), García et al. (2008), and Seifert (2018), and adding the new information for M. babiensis sp. nov. A word of caution about Myrmica microrubra Seifert, 1993. This name is not included here because its genetical isolation is not complete (Leppänen et al. 2016) and is debatable its specific status although not the existence of a highly modified, miniaturized queen morphology alongside of normal-sized queens (see an informative summary at Seifert (2018: 168–169). Biometric data in the key have been extracted from Radchenko and Elmes (2003).
Acronyms:
MiIZPAN – Collection of the Museum and Institute of Zoology, Polish Academy of Sciences, Warsaw, Poland;
MCNB – Museu de Ciències Naturals de Barcelona;
MNCN – Museo Nacional de Ciencias Naturales, Madrid, Spain;
SMNG – Senckenberg Museum für Naturkunde Görlitz, Germany;
ADC-SPC – Amonio David Cuesta-Segura personal collection, Spain;
FGPC – Fede García personal collection, Spain;
XEPC – Xavier Espadaler personal collection, Spain.
Examined material. A total of 53 specimens morphologically assignable to parasitic Myrmica have been included in this study. To carry out the species comparison, we used 11 gynes and four males of M. karavajevi, 26 gynes of M. babiensis sp. nov., and 12 specimens of the following parasitic species:
Type material.
Myrmica hirsuta Elmes, 1978
Dry mounted specimens: United Kingdom: 2 gynes labelled “England. Purbeck, Dorset. (England) Elmes leg.” “Paratype Myrmica hirsuta Elmes, 1978” [red label, printed] (XEPC).
Myrmica laurae (Emery, 1907)
Dry mounted specimens: Italy: 2 gynes of the synonym Myrmica samnitica Mei, 1987. Labelled “Abruzzo, Ovindoli. 15.VIII.1983. 1400 m. leg. M. Mei.” “Paratype Myrmica samnitica Mei, 1987” [red label, printed] (XEPC).
Non-type specimens.
Myrmica bibikoffi Kutter, 1963
Dry mounted specimen: Spain: 1 gyne from Spain. Labelled “Cava, Lleida, 23-IX-2005. F. García leg. M. bibikoffi Espadaler det.” (FGPC).
Myrmica karavajevi (Arnoldi, 1930)
Dry mounted specimens: Spain: 2 gynes labelled “Sierra de Enzia, Álava. 1000 m. 18.IV.2003, I. Zabalegui leg.” (XEPC); 3 gynes labelled “Penedos, Abadín (LU), 24-IV-2018, F. García leg. M. karavajevi F. García det.” (FGPC, XEPC).
Myrmica lemasnei Bernard, 1967
Dry mounted specimens: Spain: 2 gynes labelled “T Comes Xiques, Toses (GI), 14-V-2014, F. García leg. M. lemasnei, F. García det.” (XEPC, FGPC).
Results
Key to European parasitic Myrmica gynes
After Radchenko and Elmes (2003, 2010), García et al. (2008), and Seifert (2018), modified
1. Scape base in dorsal view gently curved, not angled (Fig. 1A) 2
–. Scape base in dorsal view angled, sometimes with additional lobe or carina (Figs 1B, 1C) 4
2. Scape shorter (SL/HL: 0.65–0.66) (Fig. 2A). Petiole triangular (Fig. 3A). Alps. Inquiline of M. lobulicornis M. myrmicoxena
–. Scape longer (SL/HL: 0.86–1.01) (Fig. 2B). Petiole rounded dorsally (Fig. 3B). Collar-like ridge in the posterior margin of the head (Fig. 4) 3
3. Base of the first gaster tergite with abundant suberect pilosity (Fig. 5A). Upper surface of postpetiole reticulate (Fig. 6A). Pyrenees. Inquiline of M. spinosior M. lemasnei
–. Base of the first gaster tergite without such suberect pilosity (Fig. 5B). Upper surface of postpetiole not reticulate (Fig. 6B). Widespread across Europe. Inquiline of the M. scabrinodis group (M. aloba, M. gallienii, M. lonae, M. rugulosa, M. sabuleti and M. scabrinodis) M. karavajevi
4. Eyes with conspicuous and appressed setae, 35–60 microns long (Fig. 7A) 5
–. Setae in the eyes absent or, if present, erect and shorter (Fig. 7B) 6
5. Scape relatively longer (SL/HL: 0.70–0.75) and with a carina at the base (Fig. 8A); postpetiole narrower (PPW/HL: 0.62–0.69). Less robust. Apenines. Inquiline of M. sabuleti or M. scabrinodis M. laurae
–. Scape relatively shorter (SL/HL: 0.61–0.69) and without carina at the base (Fig. 8B); postpetiole wider (PPW/HW: 0.70–0.89). Cantabrian Mountains. Parasite of M. aloba M. babiensis sp. nov.
6. Sculpture in the upper region of the petiolar node being longitudinally concentric, and without reticulation (Fig. 9A). Widespread across Europe. Facultative temporary parasite of M. scabrinodis M. vandeli
–. Sculpture in the upper region of the petiolar node reticulate (Fig. 9B) 7
7. Smaller species, AL < 2 mm. Frons wider (FW/HW: 0.39–0.46). Reticulation of the head mainly restricted to the rear third (Fig. 10A). Widespread across Europe. Inquiline of M. sabuleti and M. lonae M. hirsuta
–. Bigger species, AL > 2 mm. Frons narrower (FW/HW: 0.34–0.35). Most of the head reticulate, only with longitudinal striae in the frons (Fig. 10B). Western Europe. Probable temporary parasite of M. sabuleti and M. spinosior M. bibikoffi
Figure 1.
Scapes in dorsal view of gynes of: (A) M. karavajevi from Truébano de Babia; (B) M. babiensis sp. nov.; (C) M. bibikoffi from Spain.

During the first sampling (pitfall traps), we found a total of 24 dealate gynes presumably belonging to Myrmica and not attributable to any currently known species. The specimens were collected in five of the fifteen studied mountain passes (Fig. 11). These specimens presented some characters typically associated to social parasites, like a wider and higher postpetiole and abundant pilosity. During the second set of sampling (direct search), we found two gynes living inside a nest of M. aloba, confirming the parasitic affiliation of this new species. Additionally, in three sampling sites of the investigated area, we found specimens of M. karavajevi (Fig. 11): two gynes in pitfall traps and four gynes living inside the nests of M. aloba.
Species accounts
Myrmica karavajevi (Arnoldi, 1930)
(Figs 1A, 2B, 3B, 4, 5B, 6B)
New distribution data. Dry mounted specimens: Spain: Castilla y León: León: La Cueta de Babia (Cabrillanes), 43°01′N, 6°11′W, 1620 m, 1 gyne, 16-VII / 1-VIII-2010, in pitfall trap, ADC-S et al. leg. (XEPC); Sena de Luna, 42°55′N, 5°59′W, 1370 m, 1 gyne, 12-VII / 2-VIII-2010, in pitfall trap, ADC-S et al. leg. (XEPC).
Ethanol (70%) preserved specimens: Spain: Castilla y León: León: Truébano de Babia (San Emiliano), 42°56′N, 6°00′W, 1288 m, 4 gynes in one nest of M. aloba, 28-V-2022, ADC-S and Rubén Argüeso leg. One of these gynes was kept alive for six months with workers from the host nest. During that period, the colony produced alates that copulated with each other within the artificial nest. Later, four males and five gynes were stored in 70% ethanol (ADC-SPC, FGPC).
Figures 2–4.
(2) Heads in frontal view of gynes of: (2A) M. myrmicoxena, antweb specimen CASENT0907641; (2B) M. karavajevi from Truébano de Babia; (3) Petioles in lateral view of gynes of: (3A) M. myrmicoxena, antweb specimen CASENT0907641; (3B) M. karavajevi from Truébano de Babia; (4) Gyne of M. karavajevi from Truébano de Babia, posterior head border in lateral view, showing the collar-like ridge.

Myrmica babiensis García, Cuesta-Segura
& Espadaler sp. nov.
(Figs 1B, 7A, 8B, 11–17)
Etymology. The species is named after Babia, the region where most of the known localities of the new species were found. However, the Spanish phrase “estar en Babia” (“to be in Babia”) is also a colloquial expression meaning “to be absent or distracted”, what also corresponds with the fact that this species remained undetected for a very long time. Combining these aspects, we use ‘babiensis’ – a feminine gender Latin adjective derived from Babia.
Type material. Holotype: SPAIN, 1 gyne labelled “España: Castilla y León: León: La Cueta de Babia (Cabrillanes), 43°01′N, 6°11′W, 1620 m, 9-IX / 1-X-2010, trampa de caída. A.D. Cuesta-Segura, S. García Tejero, O. Pérez Fuertes, N. Pérez Hidalgo & M. Vaílez de Abajo leg.” (abbreviated below as ADC-S et al. leg.) // “MNCN_Ent 375518” // Red label “Holotipo Myrmica babiensis García, Cuesta-Segura & Espadaler, des. 2023” (MNCN).
Paratypes: All carrying a red label with “Paratipo Myrmica babiensis García, Cuesta-Segura & Espadaler, des. 2023”, all dry mounted specimens from the following localities: Spain: Castilla y León: León: La Cueta de Babia (Cabrillanes), 43°01′N, 6°11′W, 1620 m, 1 gyne, 1-VIII / 20-VIII-2010 (ADC-SPC); 1 gyne, 20-VIII / 9-IX-2010 (XEPC); 4 gynes, 9-IX / 1-X-2010 (MiIZPAN 2/2024/1, SMNG, ADC-SPC, FGPC), all in pitfall traps, ADC-S et al. leg.; La Riera de Babia (Cabrillanes), 42°58′N, 6°08′W, 1559 m, 6 gynes, 10-IX / 1-X-2010, in pitfall traps, ADC-S et al. leg. (MCNB 2023-1049, MCNB 2023-3997, MNCN_Ent 375519, MNCN_Ent 375520, ADC-SPC, FGPC); Peñalba de Cilleros (Cabrillanes), 42°55′N, 6°09′W, 1535 m, 1 gyne, 1-VIII / 21-VIII-2010 (MiIZPAN 2/2024/2); 2 gynes, 21-VIII / 12-IX-2010 (SMNG, FGPC); 1 gyne, 12-IX / 2-X-2010 (MNCN_Ent 375521), all in pitfall traps, ADC-S et al. leg.; Sena de Luna, 42°55′N, 5°59′W, 1370 m, 2 gynes, 2-VIII / 22-VIII-2010 (MNCN_Ent 375522, MCNB 2023-1050); 1 gyne, 22-VIII / 12-IX-2010 (XEPC); 2 gynes, 12-IX / 2-X-2010 (ADC-SPC, FGPC), all in pitfall traps, ADC-S et al. leg.; 1362 m, 2 gynes and 2 host workers from one nest of M. aloba, 3-X-2011, ADC-S leg. The four in one single pin (XEPC); Riolago de Babia (San Emiliano), 42°54′N, 6°05′W, 1615 m, 2 gynes, 22-VIII / 10-IX-2010, in pitfall traps, ADC-S et al. leg. (MCNB 2023-3996, MNCN_Ent 375523).
To summarize, the type specimens are deposited in the following collections: 2 gynes in MiIZPAN, 4 gynes in MNCB, 6 gynes (holotype included) in MNCN, 2 gynes in SMNG, 4 gynes in ADC-SPC, 4 gynes in FGPC and 4 gynes in XEPC.
Differential diagnosis. Myrmica babiensis sp. nov. is differentiated from M. kabylica, M. lemasnei and M. karavajevi by the smaller body size of the later species, with a mesosoma length between 1.16–1.44 mm (Radchenko & Elmes, 2003) versus 1.65–1.92 in M. babiensis (Table 1), and the lack of a collar-like ridge in the posterior occipital margin in M. babiensis sp. nov.
The shape of the scape, without caudal lobe, differentiates M. babiensis sp. nov. from M. laurae, M. hirsuta and M. bibikoffi. Moreover, compared with M. hirsuta, M. babiensis sp. nov. is more hairy and presents a less massive petiole. Also, the postpetiole is more projected ventrally in M. babiensis sp. nov. Regarding M. bibikoffi, it could be confused with M. babiensis sp. nov. in the field since both species have a big size and are very hairy. However, M. babiensis sp. nov. can be differentiated from the former one by its smaller frontal and scape indexes and its wider postpetiole (Table 1).
The species that most closely resembles M. babiensis sp. nov. is M. laurae, as both species share the same kind of pilosity in the eyes, the curved shape of the scape, and have more similar biometry (Table 1). Nevertheless, M. babiensis sp. nov. does not show any trace of a scape lobe, and the general sculpture in the body is heavier, especially in the head. Moreover, M. laurae also presents a more developed parasite syndrome, being less robust. This is particularly evident in the head, which shows a more rounded shape and more protuberant ocelli. Besides, M. babiensis sp. nov. is larger and has relatively wider postpetiole.
Since the laurae species group has been defined based on male morphology (Radchenko and Elmes 2010) and this caste is currently unknown for M. babiensis sp. nov., we have decided to refrain from assigning the new species to any species group until the males can be found and described.
Description. Gyne (Figs 12–16 and biometric measurements in Table 1). Total length of approximately 5 mm, being similar to other non-parasitic Myrmica gynes and quite large compared to most parasitic Myrmica gynes. Overall coloration light yellowish – orange. Frontal area of head, clypeus, scutellum, and some areas of the scutum and mesopleura darker. The upper surface of petiole and postpetiole also darker in some specimens. Mandibles, antennae and legs lighter. Gaster with a brownish component.
All body covered with a denser, longer, finer and more curved pilosity than in non-parasitic Myrmica gynes (Fig. 12).
Head, in frontal view, nearly as long as it is wide, with the widest line above the eyes (Fig. 13). Sculpture of the head mainly striate, and reticulate towards the occipital border. Occiput rather straight, with just a shallow concavity in the centre. Frontal lobes and frontal ridges poorly diverging. Eyes well developed, ovate but weakly elongated and hairy, with a mostly appressed pilosity 50–60 microns long (Fig. 14). Mandibles with a mean of 6.9 teeth (n=10), presenting a very small basal one. Clypeus striate longitudinally, centrally with striae curved and weaker. Anterior clypeus border convex (Fig. 13). Scape relatively short, continuously bent, and without a caudal lobe. Sometimes with visible dark line in posterior view, but not observable in other positions, without any trace of a carina (Fig. 15).
Mesosoma robust, bearing all the usual sclerites present in sexual ant individuals. Even though no winged specimen was found, M. babiensis sp. nov. is undoubtedly a flying species, as deduced from the overall mesosoma structure and the presence of tegulae and clear wing scars. In lateral view, dorsal pronotum reticulate, lateral sides with longitudinal striae. Scutum dorsally mostly smooth, with a few longitudinal striae centrally. Scutellum with longitudinal but anastomosed sculpture, in most specimens almost smooth dorsally. Spines well developed, diverging in dorsal view. The space between the spines smooth. Pectinate spurs in hind and media tibiae present, but with reduced size compared to those in M. aloba gynes.
Petiole short and high, with a well-developed anteroventral tooth. Node high and rounded, with the upper surface reticulate. Anterior face of the petiolar node straight or at most very shallowly concave. Post-petiole very wide dorsally, longitudinally sculpted, with striae curved (Fig. 16). A postpetiolar ventral process well developed, accounting for one third of the total height of postpetiole in lateral view. Gaster smooth, shiny, without appressed pubescence. Some specimens exerted a well-developed sting.
Male. Not found. All Myrmica males captured in the pitfall-traps were easily placed in known species also present in the area (see notes on natural history), none of them having characters considered to be parasitic, like reduced 12-segmented antennae, wider postpetiole or increased hairiness (Radchenko and Elmes 2003).
Distribution and notes on natural history. At present, M. babiensis sp. nov. is only known from montane pastures, between 1363 and 1620 m a.s.l. in five localities of the “Babia and Luna Natural Park” on the south side of the Cantabrian Mountains, NW Iberian Peninsula (Fig. 11). Two gynes of M. babiensis sp. nov. were found in October 2011 inside a M. aloba nest. The mixed colony was found under the layer of moss covering a stone in the middle of a meadow on a steep hillside (Fig. 17). The nest was not fully excavated, but about one hundred workers were captured. After the stereomicroscope inspection, none of the collected workers was found to bear parasitic characters. Further research will be needed to confirm whether M. babiensis sp. nov. is a workerless species or whether the presence of workers is restricted to some populations, like in M. hirsuta (Radchenko and Elmes 2010).
During the sampling of the above-mentioned five localities, we captured a total of 29 ant species using the pitfall traps (Table 2). Two additional species, found in other localities of the same project, must be added to this list of species: Myrmica ruginodis Nylander 1846, and Teleutomyrmex schneideri Kutter, 1950 (the latter recorded in Cuesta-Segura et al. 2018), and three more recorded from direct sampling: Anergates atratulus (Schenck, 1852), Chalepoxenus muellerianus (Finzi, 1922) and Chalepoxenus kutteri Cagniant, 1973 (the two latter recorded in García et al. 2017). In total, the study area hosts a total number of 34 ant species.
Figures 5–7.
(5) Petioles, postpetioles and base of the gaster in lateral view of gynes of: (5A) M. lemasnei from Spain; (5B) M. karavajevi from Truébano de Babia; (6) Petioles and postpetioles in dorsal view of gynes of: (6A) M. lemasnei from Spain; (6B) M. karavajevi from Truébano de Babia; (7) Eyes in frontal view of gynes of: (7A) M. babiensis sp. nov.; (7B) M. bibikoffi from Spain.

Figures 8–10.
(8) Scapes in dorsofrontal view of: (8A) M. laurae, antweb specimen CASENT0904098; (8B) M. babiensis sp. nov. Scales: 0.5 mm.; (9) Petioles in dorsal view of: (9A) M. vandeli worker from Spain; (9B) M. bibikoffi gyne from Spain; (10) Heads in frontal view of gynes of: (10A) M. hirsuta, antweb specimen CASENT0172757; (10B) M. bibikoffi from Spain.

Table 1.
Biometric measurements and indexes following Radchenko and Elmes (2010) of M. babiensis sp. nov., M. aloba (own data, in microns, mean ± standard deviation (minimum; maximum)) and other big sized parasitic Myrmica gynes (minimum–maximum, data taken from Radchenko and Elmes, 2003).

Discussion
Regarding the conservation status, because of the discovery of the new species in different valleys across the regions of Babia and Luna, on both sides of the Luna River, it seems that M. babiensis sp. nov. is not in an immediate danger right now. Nevertheless, in these studied mountain passes, transhumant livestock (mainly sheep and goats) graze traditionally during the favourable season, generally in summer (Loidi 2005). In recent decades, on the southern slope of the Cantabrian Mountains, there has been a significant reduction in the number of transhumant livestock, replaced by transterminant livestock (cows and horses, mainly) (Rodríguez Pascual 2001), which only feed on herbaceous plants; unlike sheep and goats that also consume woody plants (Osoro et al. 2003, Celaya et al. 2007). This traditional land use keps some areas free of bushes, which would otherwise end up as bush areas in a few decades (Morán-Ordóñez 2012), offering a completely different habitat for fauna, with a potential change of the ant community (Wiezik et al. 2013). The decrease in the livestock population, together with the replacement of the type of livestock and traditional practices, has led to the abandonment of economically unprofitable areas in the Cantabrian Mountains, resulting in an increase in scrub, which is currently mechanically eliminated in some areas and that also results in changes of bush community (Cuesta-Segura 2016).
Table 2.
Ant species captured in pitfall traps in the five locations where M. babiensis sp. nov. was found. Only workers have been taken into account, with the exception of parasitic species, where alates are also included. Localities = Loc. 1: La Riera de Babia, Loc. 2: Sena de Luna, Loc. 3: Peñalba de Cilleros, Loc. 4: La Cueta de Babia, and Loc. 5: Riolago de Babia.

Figure 11.
Distribution of Iberian parasitic Myrmica records, including Myrmica babiensis sp. nov. Red lines indicate limits of Spanish provinces. Legend of species in picture.

All specimens of M. babiensis sp. nov. were located in five mountain pastures, but four of them were abandoned or used intermittently (La Cueta de Babia; La Riera de Babia; Peñalba de Cilleros; and Sena de Luna), while only one was grazed by cows (Riolago de Babia) during the study period. Therefore, the use of these pastures and the continuity of traditional practices with livestock seem important for the conservation of the new species (Morán-Ordóñez 2020).
Like the other social parasites, we can assume their populations are scarce and patchy distributed (Tinaut and Ruano 1999). To date, M. babiensis sp. nov. meets the criteria of area of occupancy < 500 km2 and a number of locations ≤ 5 to be included as category endangered according to criteria B of the IUCN (IUCN 2022); however, without data about the extent of its population (continuing decline or extreme fluctuations) it is not possible to assign any category. However, it has to be noted that most social parasites belonging to genera Anergates Forel, 1874, Chalepoxenus Menozzi, 1923, Myrmica, Myrmoxenus Ruzsky, 1902 and Teleutomyrmex Kutter, 1950, have been included in the red lists as category vulnerable (e.g. Social Insects Specialist Group 1996a, 1996b, 1996c, 1996d, 1996e, 1996f) based on D2 criteria of the IUCN: “restricted area of occupancy or number of locations with a plausible future threat that could drive the taxon to critically endangered or extinction in a very short time” (IUCN 2022). Thus, M. babiensis sp. nov. needs to be evaluated in order to determine the degree of threat to its populations considering the data presented above.
Figures 12–16.
Myrmica babiensis sp. nov., gyne. (12) habitus of holotype in lateral view. Scale: 1 mm; (13) head of holotype in frontal view. Scale: 0.5 mm; (14) pilosity on the eyes in frontal view. Scale: 0.1 mm; (15) scape of holotype in dorsal view. Scale: 0.5 mm; (16) petiole and postpetiole of holotype in dorsal view. Scale: 0.5 mm.

Myrmica aloba is distributed in the Iberian Peninsula, the Balearic Islands and the French Pyrenees (Radchenko and Elmes 2010). It was the most frequent species of the genus in the sampling areas, and in other localities of the Cantabrian Mountains it was also among the most widespread species present in grasslands (pers. obs.). It is easily identifiable among the other Myrmica species present in the study area by the lack of a basal scape lobe and the small frontal index. Given the wide distribution of M. aloba in the Iberian Peninsula, it is remarkable that no social parasite had been associated to this species until recently (García 2018).
To date, all the records for parasitic Myrmica have been reported from the northern part of the Iberian Peninsula (Fig. 11), an area included into the Eurosiberian biogeographic region (Loidi 2017). This area is characterized by the presence of a high diversity of Myrmica, with 18 recorded species (Gómez and Espadaler 2007, Radchenko et al. 2008, García et al. 2008, Seifert et al. 2014). This number represents 66% of the Myrmica species inhabiting Western Europe (Radchenko and Elmes 2010). The opposite scenario is observed when moving southward in the Iberian Peninsula, towards the Mediterranean biogeographic region, where the richness of Myrmica species rapidly declines (Tinaut and Ruano 2021).
Regarding the complete myrmecofauna, most of the ant species in the area of Babia and Luna are Eurosiberian species distributed through most of central and northern Europe, or southern mountain ranges like the Alps (Seifert, 2018). Other ant species have more restricted distributions, being either Iberian endemics or western Mediterranean, like Lasius grandis Forel, 1909, Lasius piliferus Seifert, 1992, M. aloba and M. wesmaeli Bondroit, 1918.
Figure 17.
Habitat of Sena de Luna where two M. babiensis sp. nov. gynes were captured inside a M. aloba nest. In the foreground is the mossy stone where the parasitized colony was found.

The study area in Babia and Luna has been proved to be a hot spot of new interesting records. In this area, Lasius jensi Seifert, 1982 was recorded for the first time in the Iberian Península (Cuesta-Segura et al. 2012). Moreover, other rarely recorded species, such as Chalepoxenus muellerianus and Chalepoxenus kutteri (García et al. 2017); Teleutomyrmex schneideri (Cuesta-Segura et al. 2018); Anergates atratulus, Lasius mixtus (Nylander, 1846), Lasius umbratus(Nylander, 1846) Polyergus rufescens (Latreille, 1798), Strongylognathus testaceus (Schenck, 1852) and the two parasitic Myrmica recorded in this work, have been reported from this area. These records of social parasites found in the area represent the 24% of the total number of ant species found in “Babia and Luna Natural Park” (taking into account unpublished records for a dozen additional species), a high representation compared to 13% estimated for the entire Iberian Peninsula (Gómez and Espadaler 2007), although rather similar to other well-known Iberian regions like Burgos (18%) (García and Cuesta-Segura 2017) or Catalonia (21%) (Espadaler 2021). Notwithstanding, these findings fit with the idea of mountain habitats as potential areas for high species richness of social parasites (Tinaut et al. 2005). Accordingly, the Cantabrian Mountains expose their potential as a favourable habitat for social parasites, similarly to other mountain ranges from Europe (Alps or the Pyrenees).
Acknowledgments
We thank Sergio García Tejero, Octavio Pérez Fuertes, Nicolás Pérez Hidalgo, and Marta Vaílez de Abajo, for their contribution to field collection and segregation of the material collected during the samplings in 2010. To Marian Sánchez Gutiérrez and Rubén Argüeso Vázquez for their companionship on the field in 2021 and 2022, respectively. Our thanks to Miriam Serrano Roca for checking the Latin name and to Gema Trigos-Peral for her assistance in editing the English. To Bernhard Seifert and Sebastian Salata for their reviews and comments that improved the manuscript. The entomological material collected in pitfall traps belong to the sampling carried out under the research project IEU001A10-2 funded by Junta de Castilla y León, to which we also thank for issuing the sampling permits.
© Museum and Institute of Zoology PAS